ICEECE2012 Oral Communications Diabetes Clinical (6 abstracts)
Diabetic ketoacidosis at diagnosis influences complete remission after treatment of hematopoietic stem cell transplantation in adolescents with type 1 diabetes
W. Gu 1 , J. Hu 1 , D. Zhu 2 , W. Tang 1 , L. Li 2 , W. Cui 2 , J. Hong 1 , Y. Zhang 1 , W. Wang 1 & G. Ning 1
1Shanghai Ruijin Hospital Affiliated to Shanghai JiaoTong University, Shanghai, China; 2Nanjing Drum Tower Hospital affiliated to Nanjing University Medical School, Nanjing, China.
Objective: To determine if autologous nonmyeloablative hematopoietic stem cell transplantation (AHSCT) was worthwhile to do in type 1 diabetes adolescents with diabetic ketoacidosis at diagnosis.
Research Design and Methods: We enrolled 28 type 1 diabetes patients aged 14–30 years in a prospective AHSCT phase II clinical trial. Hematopoietic stem cells were harvested from the peripheral blood following a pretreatment consisting of a combination of cyclophosphamide and antithymocyte globulin. Changes of exogenous insulin requirement were observed and serum levels of hemoglobin A1c, C-peptide secretion during the oral glucose tolerance test (OGTT) and anti-glutamic acid decarboxylase antibody (GAD) were measured before and after AHSCT.
Results: After transplantation, complete remission (CR) defined as insulin independence was observed in 15 (53.6%, 15/28) patients for a mean period of 19.3 months over a follow-up ranging 4–42 months. The non-DKA patients achieved greater CR rate than the DKA ones (70.6%, 12/17 in non-DKA vs 27.3%, 3/11 in DKA, P=0.051). In non-DKA group, levels of fasting C-peptide, Cmax(peak value during OGTT) and AUCC(area under C-peptide release curve during OGTT) were enhanced significantly one month after transplantation and remained high during 24 months follow-up (all P<0.05). In DKA group, significant elevation of fasting C-peptide level and Cmax level were only observed at 18-month and 6-month respectively. There was no mortality.
Conclusions: We have performed AHSCT in 28 cases of type 1 diabetes. The data demonstrate AHSCT to be an effective long-term treatment of insulin dependence with greater efficacy achieved in patients without ketoacidosis at diagnosis.
Trial Registration clinicaltrials.gov Identifier: NCT00807651
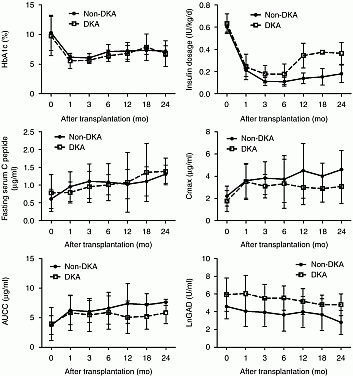
Fig 1. Time course of HbA1c, Insulin dose, fasting C-peptide, Cmax, AUCC and LnGAD level in non-DKA group and DKA group respectively Note: Cmax, peak value of C-peptide during OGTT; AUCC (area under C-peptide release curve during OGTT); LnGAD, log form of GAD because of the uneven data distribution. Solid line, non-DKA group; Dotted line, DKA group.
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research project.
Funding: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector




