ICEECE2012 Poster Presentations Pituitary Clinical (183 abstracts)
Pasireotide treatment is associated with improvements in hypertension: 12-month results from a large phase III study in Cushing’s disease
R Pivonello 1 , S Petersenn 2 , J Newell-Price 3 , F Gu 4 , M Maldonado 5 , A Trovato 5 , G Hughes 5 , L Salgado 6 , A Lacroix 7 , J Schopohl 8 & B Biller 9
1University of Naples ‘Federico II’, Naples, Italy; 2ENDOC Center for Endocrine Tumors, Hamburg, Germany; 3University of Sheffield, Sheffield, UK; 4Ministry of Health, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China; 5Novartis Pharma AG, Basel, Switzerland; 6Hospital das Clínicas, University of São Paulo Medical School, São Paulo, Brazil; 7Centre hospitalier de l’Université de Montréal, Montréal, Quebec, Canada; 8Campus Innenstadt, University of Munich, Munich, Germany; 9Massachusetts General Hospital, Boston, Massachusetts, USA.
Introduction: Patients with Cushing’s disease (CD) have an increased risk of hypertension (HTN). phase III data have shown that pasireotide leads to rapid reductions in UFC levels and significant improvements in CD symptoms. We now present further analyses of these data, evaluating the effects of pasireotide on HTN in patients with CD.
Methods: Patients with persistent/recurrent or de novo (if not surgical candidates) CD and UFC≥1.5 times the upper limit of normal (ULN) were randomized to pasireotide 600 μg (n=82) or 900 μg (n=80) sc bid. Systolic (SBP) and diastolic blood pressure (DBP) were evaluated by single measurements at baseline (n=162) and month 12 (n=78). Baseline HTN was defined as at least one of: history of anti-HTN medications; medical history of HTN; SBP>130 mmHg; or DBP>90 mmHg. Addition of medications for HTN was allowed per investigator discretion.
Results: At baseline, 77.8% of patients had HTN (n=126), of whom 97 took anti-HTN medication during the study. Mean SBP decreased from 133.5 mmHg at baseline to 126.1 mmHg at month 12 (−6.1; 95% CI: −9.8, −2.4). Mean DBP decreased from 86.3 to 82.8 mmHg (−3.7; 95% CI: −6.2, −1.2). When stratified by UFC response, these improvements were only significant in those with UFC≤ULN at month 6. Significant improvements from baseline to month 12 were seen in patients with baseline HTN (SBP: −8.0 (−12.4, −3.6); DBP: −4.7 (−7.7, −1.7)). There were no significant changes in SBP or DBP in patients without baseline HTN (SBP: 0.2 (−6.1, 6.4); DBP: −0.4 (−4.6, 3.9)). Decreases in DBP tended to be larger in patients with higher baseline DBP (Fig. 1); similar trends were seen with SBP. A >5 mmHg decrease in DBP was seen in 42% (33/78) of patients at month 12. This was higher in those with baseline HTN (50% (30/60)) than in those without (17% (3/18)). Of those with baseline HTN, a >5 mmHg decrease in DBP was seen in 63% (10/16) of patients who did not take anti-HTN medication during the study and in 46% (20/44) of those who did. Adverse events of hypotension and adrenal insufficiency were reported by 11 and 9 patients, respectively; others were as expected for a somatostatin analogue, except degree of hyperglycemia.
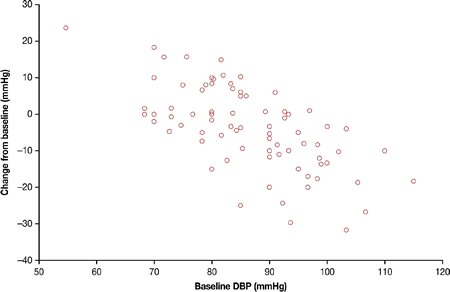
Figure 1 Changes in DBP by baseline DBP
Conclusions: In addition to rapid and sustained decreases in UFC, pasireotide treatment is associated with significant decreases in SBP and DBP in patients with CD.
Declaration of interest: I fully declare a conflict of interest. Details below:
Funding: This work was supported, however funding details unavailable.
 }
}



