SFEBES2014 Symposia Thyroid cell biology comes to your clinic (Supported by <emphasis role="italic">Journal of Molecular Endocrinology</emphasis>) (3 abstracts)
Development of functional thyroid tissue from embryonic stem cells
Sabine Costagliola
IRIBHM Université Libre de Bruxelles, Bruxelles, Belgium.
Induced overexpression of defined transcription factors has been shown to have a directing effect on the differentiation of embryonic stem cells (ESCs) into specific cell types. Nevertheless, protocols promoting coordinated self-assembly of differentiated cells into distinct morphological units with functional properties reminiscent of organs and tissues in vivo are still very sparse. Our group recently reported efficient rescue of hypothyroidism in athyroid mice transplanted with functional thyroid follicles generated from mouse ESCs in vitro. In this work, we show that an overexpression of the transcription factors NKX2.1 and PAX8 is sufficient to direct ESCs differentiation into thyroid follicular cells (TFCs) and promotes in vitro self-assembly of TFC into three-dimensional follicular structures, when associated to a subsequent TSH treatment. Cells differentiated by this protocol show significant iodide organification activity, a hallmark of thyroid tissue function. Importantly, athyroid mice grafted with ESCs-derived thyroid follicles show normalization of plasma T4 levels with concomitant decrease of plasma TSH. In addition, a full normalization of body temperature at 4 weeks after transplantation was observed. Together, these data clearly demonstrate that grafting of our ESCs-derived thyroid cells rescues the hypothyroid state and triggers symptomatic recovery along with the normalization of plasma hormone concentrations.
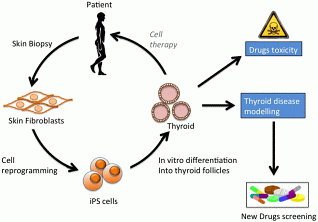
By using human pluripotent stem cells, our system would provide an unprecedented opportunity to improve our understanding of the molecular mechanisms underlying congenital hypothyroidism or to study papillary thyroid carcinoma risk allele under controlled in vitro conditions. Those thyroid diseases could be modelled, opening new therapeutic perspectives (Figure 1). One example could be the generation of functional thyroid tissue from induced pluripotent stem cells (iPSCs) derived from patients skin fibroblasts with the ultimate aim being the transplantation of iPSC-derived thyroid tissue to replace absent, ablated or damaged thyroid and restore an euthyroid state lifelong without a substitution therapy.