ECE2014 Poster Presentations Pituitary Clinical (<emphasis role="italic">Generously supported by IPSEN</emphasis>) (108 abstracts)
Efficacy and safety of lanreotide autogel (LAN-ATG) 120 mg at extended dosing intervals (EDIs) in acromegalic patients biochemically controlled with octreotide LAR (OCT-LAR) 10 or 20 mg: The LEAD study
Art-Jan van der Lelij 1 , Vyatcheslav Pronin 2 , Inga Balcere 3 , Moon-Kyu Lee 4 , Liudmila Rozhinskaya 5 , Marcello Bronstein 6 , Monica Gadelha 7 , Pascal Maisonobe 8 & Caroline Sert 8
1Department of Endocrinology, Erasmus Medical Centre, Rotterdam, The Netherlands; 2Department of Endocrinology, I. M. Sechenov First Moscow State Medical University, Moscow, Russia; 3Department of Endocrinology, Pauls Stradins Clinical University Hospital, Riga, Latvia; 4Samsung Medical Centre, Sungkyunkwan University, Seoul, Republic of Korea; 5Department of Neuroendocrinology and Bone Diseases, National Endocrinology Research Centre, Moscow, Russia; 6Neuroendocrine Unit, Division of Endocrinology and Metabolism, Hospital das Clınicas, University of Sao Paulo Medical School, Sao Paolo, Brazil; 7Endocrinology Section, Hospital Universitario Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; 8Ipsen, Boulogne-Billancourt, France.
Introduction: EDIs with LAN-ATG therapy may help improve the burden associated with long-term acromegaly management. The LEAD study evaluated the efficacy and safety of this approach by switching from OCT-LAR conventional dosing to LAN-ATG EDI.
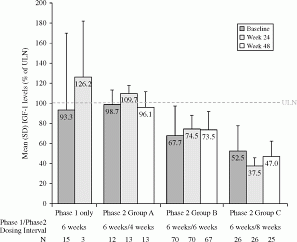
Methods: LEAD was a multinational, multicentre, open-label, non-comparative study. Acromegalic patients with normal IGF1 after OCT-LAR 10 or 20 mg injections every 4 weeks for ≧6 months were switched to LAN-ATG 120 mg at an EDI. In phase 1, patients received five LAN-ATG injections every 6 weeks for 24 weeks. In phase 2, LAN-ATG dose intervals were adjusted/maintained according to IGF1 for another 24 weeks (group A: >100 to ≤130% ULN, every 4 weeks; B: >50 to ≤100% ULN, every 6 weeks; C: ≤50% ULN, every 8 weeks). The primary endpoint was % of patients maintaining normal IGF1 on LAN-ATG EDI of 6 or 8 weeks at week 48. EudraCT:2007-005838-37; ClinicalTrials.gov:NCT00701363.
Results: The ITT population comprised 124 patients who entered phase 1 and received ≧1 dose of treatment (baseline mean age, 54.4 years, time since diagnosis, 8.7 years, OCT-LAR treatment duration, 2.5 years); 109 (88%) entered phase 2 (group A, 12%; B, 64%; C, 24%); and 107 (86%) completed the study. At week 24, 89% (83–94%) of the ITT population maintained normal IGF1 on EDI of 6 weeks. At week 48, 76% (95% CI, 68–83%) maintained normal IGF1 and had EDI of 6 or 8 weeks. IGF1 levels are shown in Figure. Over 48 weeks, treatment-emergent AEs (TEAEs) occurred in 91 (73%) patients; of these, 54 (44%) had treatment-related AEs, and only 8 (7%) had TEAEs leading to study withdrawal. GI disorders were the most common events (39 (32%)).
Conclusions: These data suggest acromegalic patients switching to LAN-ATG could continue IGF1 control without notable safety/tolerability issues; the majority achieved this while benefiting from LAN-ATG EDIs.
 }
}



