BSPED2015 e-Posters Gonadal, DSD and reproduction (8 abstracts)
The role of a next generation sequencing panel in the diagnostic pathway in disorders of sex development
Emma A Webb 1 , Vrinda Saraff 1 , Lowri Hughes 2 , S Allen 2 , Tim Cole 2 , M T Dattani 3 , I A Hughes 4 , J M W Kirk 1 , G Fews 1 & N P Krone 1
1Department of Endocrinology and Diabetes, Birmingham Children’s Hospital, Steelhouse Lane, Birmingham, UK; 2West Midlands Regional Genetics Service, Birmingham Women’s NHS Foundation Trust, Birmingham, UK; 3Paediatric Endocrinology/Genetics and genomic Medicine Programme, UCL Institute of Child health/Great Ormond Street Hospital for Children/UCL Hospitals, London, UK; 4Department of Paediatrics, University of Cambridge, Cambridge, UK.
Background: Accurate genetic diagnosis is essential in disorders of sex development (DSD), guiding medical management and enabling optimal personalized care delivery.
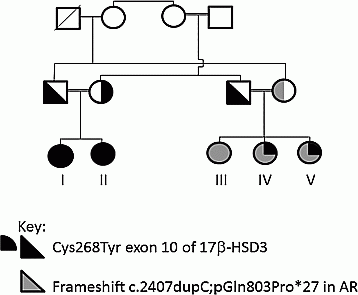
Case presentation: Two siblings (I and II) with a family history of 17β-hydroxysteroid dehydroxygenase (17β-HSD3) deficiency presented postnatally with isolated labial swelling. Karyotype was 46,XY and urinary steroid profile (USP) normal. HCG-stimulated testosterone/androstenedione ratio was 0.17 (normal >0.8) in patient II (unavailable patient-1). HSD17B3 was sequenced. A homozygous missense p.Cys268Tyr mutation was identified in both children.
Patient V presented aged 1 week with bilateral inguinal masses. External genitalia appeared female, karyotype was 46,XY and USP was normal. Stimulated testosterone/androstenedione ratio was 3.27. Gonadectomy was performed at 18 months. Her siblings were referred for assessment. Both had female external genitalia, 46,XY karyotype, normal USP and baseline androstenedione concentrations that did not rise post HCG-stimulation. Patients IV and V were heterozygous for the HSD17B3 p.Cys268Tyr mutation, whereas patient III was not a carrier. Targetted sequencing was therefore performed using a custom-designed TruSeq amplicon panel covering 32 genes associated with 46,XX and 46,XY DSD. A novel hemizygous frameshift mutation c.2407dupC; p.Gln803Profs*27 in the androgen receptor was identified in patients III–V.
Conclusion: These cases highlight the value of targeted sequencing panels in DSD. Biochemical tests can be equivocal, for example, performing a USP is not helpful in prepubertal children with 17β-HSD3 deficiency. Reaching the correct diagnosis in DSD is critical to subsequent management decisions, e.g. adult gonadectomy facilitates a more physiological transition through puberty in CAIS, whereas early gonadectomy or a block and replace regimen during puberty is required in patients with 17β-HSD3 mutations. Although a second hit was thought unlikely in patient V, high throughput sequencing is increasingly leading to the identification of patients with mutations in more than one gene, highlighting the need for periodic review of molecular testing results.
 }
}



