SFEBES2012 Poster Presentations Obesity, diabetes, metabolism and cardiovascular (73 abstracts)
Third agents in treatment of diabetes mellitus, patient motivation and weight reduction
Ahmed Siddiqi
Diabetes and Endocrinology, Southend University Hospital, Southend, United Kingdom.
Aim: To review the local clinical practice of use of third agent in the treatment of type 2 diabetes and comparison of clinical efficacy of available third agents and patient motivation with these agents. Method: We used the computerized records of type 2 diabetic patients who were started on third pharmacological agent (tablets, insulin, exenatide or liraglutide) or GLP-1 agonists which may not be the third agent and followed them up for 6 months. Medical notes were used if all the information was not available in the computerized database.
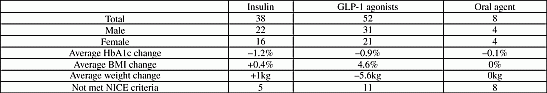
Results: Out of a total of 98 patients 53.1% were prescribed GLP-1 agonists, 38.7% insulin and 8.2% oral agents. HbA1c reduced by 1.2% with insulin, 1.5% with exenatide, 0.67% with liraglutide and 0.1% with oral agents. Body mass index (BMI) was increased by 0.1(0.1%) with insulin, reduced by 1.9(5%) with exenatide, reduced by 1.6(4.1%) with liraglutide and no change with oral agents. 21.4% got their third oral agent changed to an injectable agent. Clinic attendance was 99% with exenatide, 100% with liraglutide, 64% with insulin and 38% with oral agents. 23% of patients who were prescribed GLP-1 agonists did not meet NICE criteria. The patients who did not fulfill the NICE criteria had a reduction of 1.3% in their HbA1c and 2.5% in their BMI. These results were comparable to ABCD national audit data.
Conclusion: GLP-1 agonists were the most and oral agents were the least clinically effective third agents in the treatment of type 2 diabetes. GLP-1 agonists cause HbA1c reduction comparable to insulin but in addition to this it helps with significant weight reduction and patients’ motivation. It will be worth considering relaxing NICE criteria to allow more patients to be treated with GLP-1 agonists which will help in weight reduction leading to reduction in obesity related complications.
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding: No specific grant from any funding agency in the public, commercial or not-for-profit sector.
Third Agents’ Comparison
 }
}



