ICEECE2012 Poster Presentations Pituitary Clinical (183 abstracts)
Pasireotide treatment is associated with clinically meaningful improvements in health-related quality of life in Cushing’s disease: results from a large, randomized, double-blind phase III trial
S Webb 1 , X Badia 2 , W Zgliczynski 3 , L Portocarrero-Ortiz 4 , M Maldonado 5 , A Trovato 5 , A Forsythe 6 , L Nelson 7 , L McLeod 7 , C De Block 8 & M Gadelha 9
1Hospital Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain; 2IMS Health, Barcelona, Spain; 3Centre for Postgraduate Medical Education, Warsaw, Poland; 4National Institute of Neurology and Neurosurgery ‘Manuel Velasco Suárez’, Mexico City, Mexico; 5Novartis Pharma AG, Basel, Switzerland; 6Novartis Pharmaceuticals Corporation, Florham Park, New Jersey, USA; 7RTI Health Solutions, Research Triangle Park, North Carolina, USA; 8University Hospital, Antwerp, Belgium; 9Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Introduction: Patients with Cushing’s disease have significantly impaired health-related quality of life (HRQoL). Effective treatment is needed to treat Cushing’s disease and improve HRQoL; however, there are currently no approved medical treatments for Cushing’s disease. The effect of pasireotide on HRQoL in patients with Cushing’s disease was evaluated as part of a randomized, phase III study.
Methods: Patients with persistent/recurrent or de novo (if not surgical candidates) Cushing’s disease and UFC levels≥1.5 times the upper limit of normal (ULN) were randomized to pasireotide 600 μg (n=82) or 900 μg (n=80) SC bid. Dose titration (max: 1200 μg bid) was allowed after month 3. UFC control was defined as UFC≤ULN, partial control as UFC>ULN and≥50% reduction from baseline, and uncontrolled as UFC>ULN and <50% reduction from baseline. HRQoL was assessed at baseline and months 3, 6 and 12 using the 12-item CushingQoL questionnaire. Items were scored on a 5-point scale, resulting in a score of 12 (worst) to 60 (best) standardized to 1–100. A change in CushingQoL score of >10.1 has been estimated to be clinically meaningful.
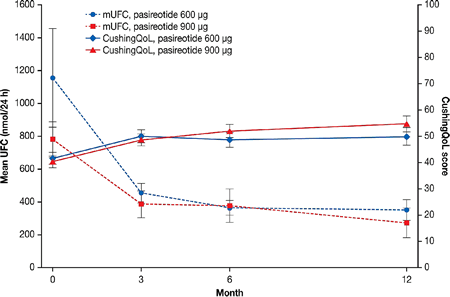
Figure 1 Mean UFC and CushingQoL scores
Results: Overall, HRQoL increased from 41.1 at baseline (n=159) to 52.5 at month 12 (n=76) (mean increase: 11.1; 95% CI: 6.8, 15.5). In both dose groups, HRQoL improved from baseline along with rapid and sustained decreases in UFC levels (Fig.1). There was a clinically meaningful improvement in HRQoL at month 12 in patients whose UFC levels were controlled (n=29; mean improvement: 12.8; 95% CI: 7.1, 18.5) or partially controlled at month 6 (n=17; mean improvement: 10.7; 95% CI: 0.8, 20.5), but the improvement in HRQoL did not reach the 10.1 change threshold in the uncontrolled group (n=30; mean improvement: 9.9; 95% CI: 2.3, 17.6). The greatest HRQoL improvements (≥20 points) were seen in patients with the greatest UFC decreases (from >10xULN to ≤5xULN; n=5). Significant correlations (P<0.01) were seen between changes in CushingQoL scores and changes in mean UFC (r=−0.40; n=68), BMI (r=0.31; n=74), weight (r=0.32; n=74), and Beck depression inventory score (r=−0.59, n=72).
Conclusion: Pasireotide treatment decreases UFC level and improves HRQoL. Clinically meaningful improvements in HRQoL are seen regardless of achieving fully normalized UFC.
Declaration of interest: I fully declare a conflict of interest. Details below:
Funding: This work was supported, however funding details unavailable.
 }
}



