NuclearReceptors2018 Invited Speaker (1) (14 abstracts)
Rapid, cytoplasmic actions of the glucocorticoid receptor impact on cell movement
Stephen Kershaw , David J Morgan , Andrew Brass , James Boyd , David Spiller , Egor Zindy , Mudassar Iqbal , Laura C Matthews & David W Ray
University of Manchester, Manchester UK.
Glucocorticoids (GCs) act through the glucocorticoid receptor (GR) to regulate immunity, energy metabolism, and tissue repair. The inactive GR is held in the cytoplasm in a multi-protein complex, which upon ligand binding undergoes a conformational change. Activated GR translocates to the nucleus to regulate gene expression (over hours), but some effects occur more rapidly. GCs inhibit cell migration through an uncertain mechanism. We now show a very rapid effect, and surprisingly find the GR agonist Dexamethasone, and antagonist, RU486, are equipotent. The migration effect was prevented by GR knockdown, confirming GR specificity, but not by actinomycin D treatment, suggesting a non-transcriptional mechanism. To investigate the GC effect we analysed microtubule network kinetics using plus end microtubule real time assays, which revealed increased tubulin acetylation –a marker of microtubule stability. Inhibition of the cytoplasmic deacetylase HDAC6, which deacetylates tubulin, mimicked the GC effect, and HDAC6 overexpression rescued the GC effect, implicating HDAC6 as the GC effector. We found interaction between GR and HDAC6, using fluorescent cross correlation spectroscopy, and showed HDAC6 intranuclear mobility restriction following GC treatment. We propose that activated GR inhibits HDAC6 deacetylation of microtubules, increasing stability of the microtubule network and reducing cell motility.
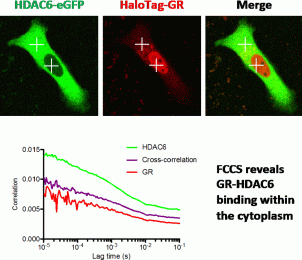
We therefore report a novel, non-transcriptional mechanism whereby GR agonists and antagonists, through actions on HDAC6, rapidly reorganize cell architecture to change cell function.

DOI: 10.1530/endoabs.54.IS5



