BES2019 BES 2019 Low dose continuous IV etomidate for severe Cushing’s syndrome in the non-critical care setting: a clinical study (1 abstracts)
Low dose continuous IV etomidate for severe Cushing’s syndrome in the non-critical care setting: a clinical study
SM Constantinescu 1 , A Lefebvre 1,2 , RM Furnica 1 & D Maiter 1
1Division of Endocrinology, Cliniques Universitaires Saint-Luc, 1200 Brussels, Belgium; 2Division of Endocrinology, Clinique Saint-Pierre, 1340 Ottignies, Belgium.
Introduction: Severe Cushing’s syndrome (CS) is defined by extremely high serum cortisol levels (usually >1000 nM), along with severe and deadly complications, including sepsis, heart failure, acute psychosis, vascular thrombosis, digestive haemorrhage and bowel perforation. Rapid control of such hypercortisolism is mandatory and has been shown to decrease operative mortality and complications. Oral treatments such as ketoconazole and metyrapone are often inadequate in the face of life threatening complications and limited oral intake possibilities. Etomidate is a hypnotic agent widely used in general anaesthesia induction, while also being one of the most potent inhibitors of 11-B-hydroxylase and therefore of cortisol secretioni. Low dose continuous intravenous (IV) infusion of etomidate has been shown to rapidly decrease cortisol levels in patients with severe CS in the ICU settingiiiii, without causing harmful side effects (myoclonus or adrenal crisis) A safe use of etomidate has been also suggested for similar indication in the non-ICU settingiv.
Aim of the work: We retrospectively analysed the efficacy and safety of low dose IV etomidate infusion in five patients with severe and complicated CS treated in our (non ICU) Endocrinology unit between 2012 and 2018.
Main results: All five patients were male, with an average age of 53.4 years, and all had cancer: Three had cortisol secreting adrenal carcinomas and two had ectopic ACTH secretion from a malignant neuro-endocrine tumour. All patients had several complications before treatment, including infections, vascular thrombosis, cardiac failure, severe hypertension and/or uncontrolled diabetes. Median cortisol levels on admission were 1288 nM (range 751–2301 nM) (Figure 1). Etomidate (Hypnomidate® 20 mg/10 ml propylene glycol solution, Janssen pharmaceuticals) was administered through a peripheral IV catheter at a starting dose of 0.03 mg/kg/h. Infusion rate was then adjusted by 0.5 mg/h increments in order to achieve morning cortisol levels between 200 and 300 nM. Mean effective etomidate rate was 3 mg/h and the average number of days before reaching cortisol levels <500 nM was 4.2. Median morning cortisol level upon discontinuation of etomidate was 306 nM (range 183–479 nM). No adverse effect related to treatment was noted (in particular no somnolence) and no propylene glycol related adverse effects were noted (no renal failure, phlebitis, or haemolysis). Four out of five patients survived after etomidate discontinuation, being subsequently treated by primary tumor resection, mitotane and/or bilateral adrenalectomy. The single patient who died had a 16 cm adrenal tumour with peritoneal carcinomatosis and a poor performance status (ECOG 3–4) that precluded surgery or chemotherapy. The decision was made, together with the patient and his family, to stop etomidate and enter palliative care.
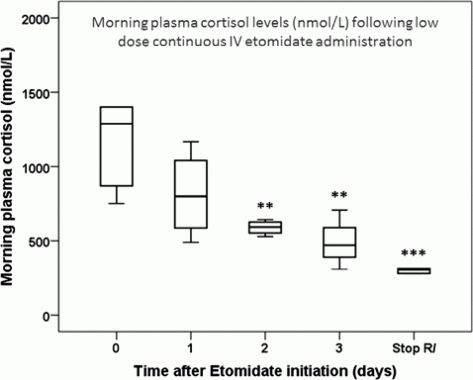
Figure 1.
Conclusion: We report a case series of 5 patients treated in a non-intensive care unit with low dose continuous IV etomidate for severe and complicated Cushing’s syndrome. Treatment was rapidly successful in reducing cortisol levels below 500 nmol/l within 3–5 days and did not result in any treatment related adverse effects.
 }
}



