BES2019 BES 2019 A case of meningioma after cyproterone acetate use (1 abstracts)
A case of meningioma after cyproterone acetate use
P Chasseur 1 , M Bruneau 2 & B Corvilain 1
1Endocrinology Unit, Erasme hospital, Université Libre de Bruxelles, Anderlecht, Belgium; 2Neurosurgical Unit, Erasme hospital, Université Libre de Bruxelles, Anderlecht, Belgium.
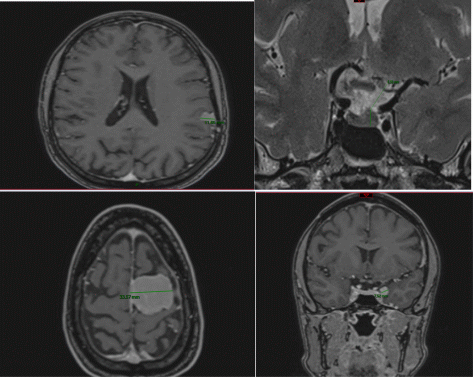
A 43-year-old roman, previously living in France was referred to the endocrinology department for Hashimoto’s hypothyroidism in 2017. Thyroid function was easily normalized by a treatment by thyroxine. She had also a medical history of severe alopecia since the age of 20. The diagnosis of androgenetic alopecia was made at that time by a French dermatologist and treatment by estradiol valerate 2 mg with cyproterone acetate (CPA) 50 mg was started. The dose of CPA was lowered to 25 mg 10 years later, in association with finasteride 5 mg, minoxidil 5% and an oral contraceptive pill (ethynilestradiol 0.02 mg and drospirenone 3 mg). In December 2018, she reported atypical headaches concomitantly with a stress episode. She had also a slight elevation of the prolactin level. Despite the atypical nature of headaches, a brain MRI was proposed in view of the prolactin level and the recent concerns about the risk of meningioma in patients with a long term treatment by CPA. Brain MRI was performed, revealing incidental discovery of 4 meningiomas in the left cerebral hemisphere. (34 mm, 19 mm, 13 mm and 7 mm). CPA and oral contraceptive pill were stopped. The brain surgeon confirmed the lack of indication for immediate surgery. In order to evaluate the evolution of the lesions after stopping anti-androgenic treatment a control brain MRI is scheduled for October 2019. Meningiomas are benign tumors, with a clear female predominance, arising from the meningothelial cells of the arachnoid membrane (20% of the intracranial tumors). Progesterone receptor responsiveness to sex hormones has been hypothesized to promote the development of meningioma. The relationship between meningiomas and CPA is now well documented. CPA is a progestin-like drug with a strong anti-androgenic effect, used to treat androgen-related alopecia in women, but also, at higher doses, inoperable prostate carcinoma and transgender women (male to female). Risk of meningioma among users of CPA was evaluated in three cohort studies.1,2,3 A retrospective cohort study performed in Spain demonstrated that patients (70% female, 30% male) using high dose of CPA (50 mg or more) had an incidence rate of 60/10000 person-years (6.6 person/10000 -years in the control group), with a rate ratio of 11.4 after adjusting for age and gender. Among the low dose CPA users, no meningioma was identified.1 Risk of meningioma was also significantly higher in a study cohort performed in the Netherlands, evaluating occurrence of brain tumors in adult transwomen (male to female) taking cross-sex treatment consisting of high dose CPA (50–100 mg). The median person-time of observation was 6.22 years. Standardized incidence ratio (SIR’s) of meningiomas were 4.1 compared with the incidence rate of a European female population and 11.9 compared with an European male population.2 Finally, a UK cohort study (1996–2008) found out a significant increased risk of meningioma among male current users of high-dose CPA (>50 mg) compared with no-users (Odds-ratio of 6.30). No significantly increased risk was found among past users (most recent prescription ended more than 1 year before index date) or among low dose CPA users.3 Evolution of meningiomas after stopping CPA was evaluated by a French series of 12 patients taking CPA for a long period (8–30 years) at an average dose of 40 mg. After stopping CPA, tumor shrinkage was observed in 11 out of 12 cases within a mean period of 5 months and there was no regrowth of the tumor in any cases during a mean 12-month follow up, which confirms that medication withdrawal followed by observation is the first line of treatment.5 There a no clear recommendations on the follow up needed in patients treated by CPA. A review on the safety of CPA is presently being carried out by the EMA. As CPA is mainly prescribed in France, the only recommendations presently available for the follow up are those of the “agence nationale de sécurité du médicament” based on very limited data:
• Prolonged use of CPA at high dosage should be avoided if possible (risk of meningioma multiplied by 7 for a 6-month treatment and by 20 for a 5-year treatment at a dose > 50 mg);
• MRI should be performed at the beginning of treatment for all patients;
• In case of continuation of treatment, MRI will be renewed at 5 years then every 2 years if the MRI at 5 years is normal;
• In patients who have stopped treatment, it is not necessary to perform brain imaging in the absence of clinical signs;
• If meningioma is found, the treatment must be stopped permanently. Neurosurgical advice is recommended – conservative treatment is often possible as meningiomas are regressing or stabilizing after stopping treatment;
• The need for treatment by CPA must be reassessed for each patient;
In conclusion, we report the case of a 43-year-old woman with multiple meningiomas occurring after a 23-year-treatment with high dose of CPA prescribed for androgenetic alopecia. Treatment was stopped and we adopted a conservative attitude with a follow-up brain MRI scheduled on October 17th 2019…
References: 1. Gil M, Oliva B, Timoner J et al. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: evidence from a population-based cohort study, British Journal of Clinical Pharmacology, 72:6 (2011), 965–968.
2. Nota N, Wiepjes C, de Blok C et al. The occurrence of benign brain tumors in transgender individuals during cross-sex hormone treatment, Brain 2018: 141; 2047–2054.
3. Cea-Soriano L, Blenk T, Wallander M-A et al. Hormonal therapies and meningioma: is there a link? Cancer epidemiology 36 (2012); 198–205.
4. Portet S, Naoufal R, Tachon G, et al. Histomolecular characterization of intracranial meningiomas developed in patients exposed to high-dose cyproteron acetate: an antiandrogen treatment., Neuro-oncology advances, 20 (2019); 1–12.
5. Bernat A-L, Oyama K, Hamdi S et al. Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: a case series of 12 patients, Acta Neurochir 157 (2015): 1741–1746.
 }
}



