BES2019 BES 2019 Severe hypocalcemia after a single denosumab injection and tumor-induced persistent hypophosphatemia in a patient with metastatic prostate cancer (1 abstracts)
Severe hypocalcemia after a single denosumab injection and tumor-induced persistent hypophosphatemia in a patient with metastatic prostate cancer
Theunissen Aude 1 , Roumeguere Thierry 2 , Corvilain Bernard 3 & Kyrilli Aglaia 3
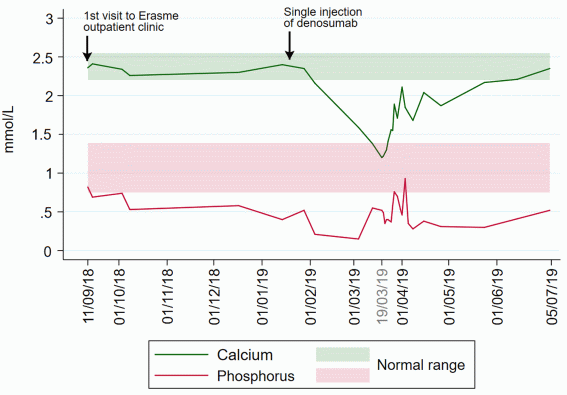
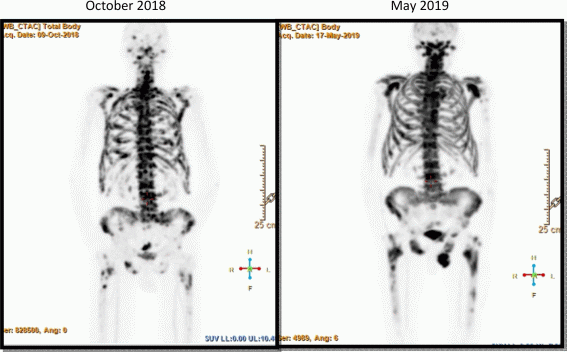
Case report: A 81-year-old male patient suffering from a Gleason 9 pT3aN0M0R1 prostatic adenocarcinoma was treated with radical prostatectomy and adjuvant radiotherapy in 2014. He presented in outpatient clinic for lumbar pain in October 2018. A 18F-NaF PET/CT showed diffuse metastatic bone involvement. Laboratory results showed hypophosphatemia (0.52 mmol/L, N: 0.75–1.39), normal renal function, normocalcemia (2.35 mmol/L, N: 2.20–2.55), normal 25-OH vitamin D (36 μg/L, N: 30–80), elevated total alkaline phosphatase (ALP) (1211 U/L, N: 56–119) and elevated prostate-specific antigen (PSA) (940 ng/ml, N<6.5 μg/L). Castration treatment with a GnRH antagonist was initiated. A single denosumab (anti-RANKL antibody) injection was administered on January 18 2019 in combination with 1200 mg of phosphorus-element supplementation. 32 days after denosumab injection, he presented with severe symptomatic (limb paresthesia and QT prolongation at 510 ms on electrocardiogram) grade 3 hypocalcemia at 1.59 mmol/L and grade 4 hypophosphatemia at 0.15 mmol/L, requiring hospitalization. The other blood tests showed: elevated serum parathyroid hormone (PTH) (125 ng/dl, N < 49), high serum levels of bone formation markers; osteocalcin at 46 μg/L (N: 14–46) and bone ALP at 91 μg/L (N: 5.5–22.9), low serum bone resorption marker; C-Telopeptide at 0.06 μg/L (N: 0.1–0.6), suppressed calciuria (<0.12 mmol/l), a paradoxical increase in phosphaturia (54 mmol/L) compared to serum hypophosphatemia, normal 25-OH vitamin D (50.4 μg/l), low 1,25-OH vitamin D (23.8 ng/l, N: 29–83.6) and increased serum levels of fibroblast growth factor 23 (FGF23) (393 pg/mL, N: 23–95). The patient required administration of intravenous (IV) calcium gluconate up to 8 g per day in addition to oral calcium citrate 6 g per day, IV and oral phosphorus and alphacalcidol at 2 μg per day for 14 days to resolve symptoms and normalize QT interval. He was discharged with persistent hypocalcemia (2.04 mmol/L) and hypophosphatemia (0.28 mmol/L). Serum calcium and PTH levels eventually normalized in June 2019 on ongoing oral supplementation, 6 months after denosumab injection, while hypophosphatemia is persistent to date. Metastatic bone disease showed progression at the last oncological evaluation in May 2019.
Discussion and physiopathological explanation: In our patient, suffering from high-burden metastatic bone disease, severe hypophosphatemia preexisted to denosumab injection and persists to date. It is probably caused by high serum FGF23 levels and low active 1,25 OH vitamin D indicated tumor-induced osteomalacia as a result of tumor FGF23 hypersecretion. The action of FGF23 on phosphocalcic metabolism is essentially due to its phosphaturic action with no direct effect on calcium metabolism. Severe hypocalcemia, despite increased serum PTH levels, suppressed calciuria and IV calcium administration at high doses, was attributed to denosumab itself which is a potent inhibitor of osteoclast-mediated bone resorption leading to highly increased influx of calcium into bone. Denosumab-induced hypocalcemia is more frequently described in case of vitamin D deficiency and impaired renal function that were not present in our patient. However, male sex, osteoblastic metastases, high serum PSA levels and high bone turnover, as in the case of our patient, have been recently shown to increase the risk for denosumab-related hypocalcemia.
Conclusion: This case illustrates that patients with castration-resistant metastatic prostate cancer should be carefully monitored in clinical practice for potentially severe calcium-phosphate homeostasis disorders: We observed in this patient a persistent hypophosphatemia induced by FGF23 hypersecretion and a denosumab-induced hypocalcemia. This case illustrates that in the context of high-burden bone disease, one single injection of denosumab may induce severe hypocalcemia even in the absence of vitamin D deficiency or renal function impairment. Whether increased secretion of FGF23 is a risk factor for Denosumab-induced hypocalcemia is not addressed in the literature but it is well known that FGF23 inhibits renal production of 1,25-dihydroxyvitamin D and at least in patients with normocalcemia conditions an inhibitory effect on PTH secretion.

Figure 1 Calcemia and phosphatemia during the time.

Figure 2 Images of the A18F-NaF PET/CT.
References: 1. Body J-J, von Moos R, Niepel D et al. Hypocalcemia in patients with prostate cancer treated with bisphosphonate or denosumab: prevention supports treatment completion. BMC Urology. 2018;18:81.
2. Mace L, Gravesen E, Nordholm A et al. Fibroblast growth factor (FGF) 23 regulates the plasma levels of parathyroid homone in vivo through the FGF receptor in normocalcemia, but not in hypocaclemia. Calcified Tissue International. 2018;102:85–92.
3. Kinoshita Y, Arai M, Ito N et al. High serum ALP level is associated with increased risk of denosumab-related hypocalcemia in patients with bone metastases from solid tumors. Endocrine Journal. 2016;63(5),479–484.
4. Lee E, Riesco Martinez M, Blakely K et al. FGF23: Mediator of poot prognosis in a sizeable subgroup of patients with castration-resistant prostate cancer presenting with severe hypophosphatemia? Medical Hypothesis. 2014;83: 482–487.
 }
}



