BES2024 BES 2024 CLINICAL CASE REPORTS (10 abstracts)
Case report: misleading increase in thyroid- stimulating hormone receptor antibodies during pregnancy in graves disease: value of functional, cell-based assays
Astrid Claessens 1,2 , Katrien Clotman 1 , Jan Monballyu 1 & Evy De Witte 3
1Department of Endocrinology, AZ Klina, Brasschaat.2Faculty of Medicine, KU Leuven, Leuven.3Department of Clinical Biology, AZ Klina, Brasschaat
Introduction: Graves disease is an autoimmune endocrinopathy caused by thyroid-stimulating hormone receptor antibodies (TSH-R-Ab). Classically, this results in hyperthyroidism as thyroid-stimulating autoantibodies (TSAb) predominate. Within the spectrum of autoimmune thyroid disease, hypothyroidism in the context of thyroid-blocking autoantibodies (TBAb) is rare. In addition, TSH-RAb can also exhibit neutral activity1. The net activity of the antibodies determines the clinical presentation and approach. Specific consideration and monitoring in the context of pregnancy is suggested due to TSH-R-Ab’s ability to migrate transplacentally and affect the fetus’ thyroid gland. However, conventional thyroid receptor binding tests detect all types of thyroid hormone receptor antibodies and cannot differentiate between them. The value of functional, cell-based, assays in an atypical course of Graves disease is demonstrated in this case report.
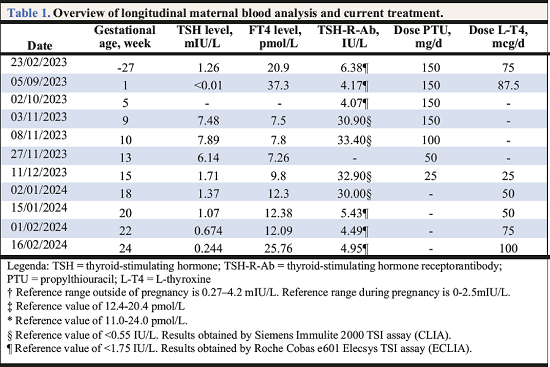
Case report: A 36-year-old woman with Graves disease without ocular involvement was referred to us because of an increasing TSH-R-Ab titer and paradoxical evolution towards hypothyroidism during twinpregnancy (Table 1). She had been treated for 56-months with propylthiouracil (PTU) along with LT4replacement therapy according to the block-replacement-regimen. The TSH-R-Ab titer, measured with two different chemiluminescence immuonoassays during follow-up, remained persistently elevated. However, from the gestational age of 10 weeks, we noticed a tendency toward hypothyroidism and a remarkable increase in TSH-R-Ab titer, taking into account the difference in immunoassay. The quantity and functional properties of TSH-R-Ab can be altered during gestation due to immunological changes. In classical Graves caused by TSAb, these antibodies will usually decrease during pregnancy. However, the antibodies may become blocking2 or neutral in rare cases. In consideration of hypothyroidism, the dose of PTU was reduced incrementally. L-T4 was associated given the low-tonormal T4-titer, where we aimed for higher values to sustain neurological fetal development. Ultimately, PTU was stopped and L-T4 progressively increased according to thyroid testing every two weeks. Development of both fetuses was normal with no evidence of hypo- or hyperthyroidism. Considering the appearance of TBAb would imply a risk of hypothyroidism in the fetuses, a functional TSH-receptor-antibody assay was requested to differentiate between all types of TSH-R-Ab3. This test was performed by Prof. Dr. Kahaly at Johannes Gutenberg University (Mainz, Germany). We concluded that neither thyroid-stimulating nor thyroid-blocking antibodies were present. Therefore, the measured antibodies were neutral. A favorable result given that these antibodies do not affect thyroid function, neither the mother’s nor the fetuses’. Since this was a twin pregnancy complicated with atypical Graves hyperthyroidism, further multidisciplinary management and postpartum monitoring of the neonates’ thyroid function is indicated.
Conclusion: When observing a switch between hyper- and hypothyroidism in patients with known Graves disease, it is important to screen for functional TSH-R-Abs. This distinction is important during pregnancy because inadequate maternal L-T4 levels could irreversibly impair fetal neurological development. Other indications for screening could include Graves disease with positive TSHR-A-bs persisting after 18 months of block-replacement therapy, before pregnancy when there is a known history of auto-immune thyroid disease addressed with thyroidectomy4 or radioactive iodine and autoimmune thyroid dysfunction secondary to immune reconstitution therapy5.
Attachment
References: 1. Antonelli A. Graves’ disease: Clinical manifestations, immune pathogenesis (cytokines andchemokines) and therapy. Published online 2020. doi:10.1016/j.beem.2020.101388.
2. Kung AWC, Jones BM. A Change from Stimulatory to Blocking Antibody Activity in Graves’ Disease during Pregnancy*. Published online 1998. Accessed February 11, 2024. https://academic.oup.com/jcem/article/83/2/514/2865426.
3. Diana T, Li Y, Olivo PD, et al. Analytical Performance and Validation of a Bioassay for Thyroid-Blocking Antibodies. Thyroid. 2016;26(5):734-740. doi:10.1089/THY.2015.0447.
4. Dierickx I, Billen J, Vanhole C, et al. Journal of Obstetrics and Gynaecology Severe fetal andneonatal hyperthyroidism years after surgical treatment of maternal Graves’ disease. Severe fetal and neonatal hyperthyroidism years after surgical treatment of maternal Graves ’ disease. Published online 2014. doi:10.3109/01443615.2013.831044.
5. Muller I, Moran C, Lecumberri B, et al. 2019 European Thyroid Association Guidelines on the Management of Thyroid Dysfunction following Immune Reconstitution Thera
 }
}



