Theranostics2016 4th Theranostics World Congress 2016 Innovative Theranostics (17 abstracts)
Critical aspects of radiochemical purity determination of 68Ga-BCA-peptides and behavioral features of different 68Ga species
Anton A Larenkov & Alesya Ya Maruk
Burnasyan Federal Medical Biophysical Center, Moscow, Moscow, Russia.
Determination of radiochemical purity (RCP) of radiopharmaceuticals based on 68Ga-BCA-peptides (68Ga-DOTA-TOC/-TATE, 68Ga-NODAGA-RGD2, 68Ga-PSMA-HBED-CC, etc.) is an extremely important part of QC in routine clinical practice. Nature of gallium leads to formation of two major radiochemical impurities in the radiopharmaceutical: hydrolyzed 68Ga (colloid) and unbounded ionic forms of 68Ga. The amounts of each impurity and 68Ga-BCA-peptide are strictly normalized in accordance with pharmacopoeial standards. In 8th EuPh there is no method allowing to separate all of radiochemical impurities in 68Ga preparations. Normally it takes to use combination of two systems to separate them (iTLC-SG and 1M ammonium acetate/methanol 50:50 (V/V) and HPLC or iTLC-SG–citric buffer).
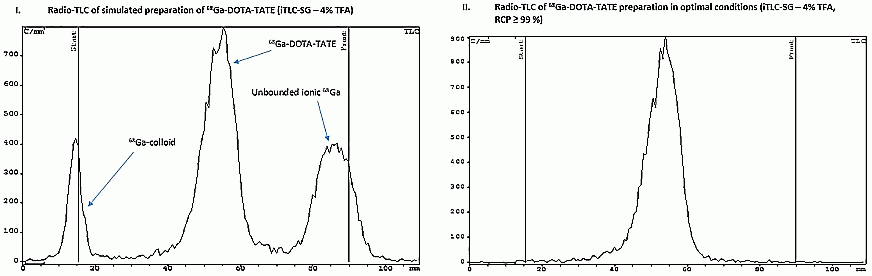
In the present study, effective iTLC method for the determination of RCP of 68Ga-radiopharmaceuticals was developed (with no double-developing, changing of eluents or additional manipulation). In this method iTLC-SG strips and commonly used eluent TFAaq. (3–5% (V/V)) are used. The method allows determining each of the key radiochemical forms of 68Ga (colloid, bound, free ionic) separately with the peaks separation being no less than 7–8 σ. Rf =0.0–0.1 for 68Ga-colloid; Rf=0.5–0.6 for 68Ga-BCA-peptides; Rf =0.9–1.0 for ionic 68Ga:
The method is simple and fast: for developing of 100 mm strip only 4–6 min is required (versus 18–20 min for pharmacopoeial method). The combination of typical chromatographic systems mentioned above as well as gel-electrophoresis and SPE were used as control.
The method has been tested on various compounds (including 68Ga-DOTA-TOC/-TATE, 68Ga-NODAGA-RGD2, etc.) and mixtures with different values of RCP. It was found, that content of 68Ga-colloid and ionic 68Ga(III) determined with the method developed correlates with control results very well. It also was found, that in some cases HPLC shows wrong RCP values (erroneously high) not only due to presence of 68Ga-colloid, but due to presence of other 68Ga-ionic species as well.
 }
}



