ECE2019 Oral Communications Reproduction 1 (5 abstracts)
The neurokinin 3 receptor antagonist, fezolinetant, is effective in treatment of menopausal vasomotor symptoms: responder analysis results from a randomized, placebo-controlled, double-blind, dose-ranging study
Graeme Fraser 1 , Samuel Lederman 2 , Arthur Waldbaum 3 , James Young 4 , Laurence Skillern 5 & Steven Ramael 1
1OGEDA SA, a wholly owned subsidiary of Astellas Pharma, Inc., Gosselies, Belgium; 2Altus Research, Lake Worth, USA; 3Downtown Women’s Healthcare, Denver, USA; 4Astellas Pharma, Inc., Northbrook, USA; 5Astellas Pharma Europe Ltd, Chertsey, UK.
Background: The brain’s thermoregulatory center is stimulated by activation of neurokinin 3 receptor (NK3R) and inhibited by estrogen. In menopause, declining estrogen disturbs this balance, producing vasomotor symptoms (VMS). This study assessed response to the NK3R antagonist fezolinetant in women with menopausal VMS.
Methods: This phase 2b, double-blind trial (NCT03192176) randomized menopausal women (age >40−65 y) with moderate/severe VMS (≥50/wk) to fezolinetant 15, 30, 60, or 90 mg BID or 30, 60, or 120 mg QD or placebo for 12 weeks. Response and percentage reduction in frequency of moderate/severe VMS were prespecified secondary endpoints. Response was defined as ≥50%, 70%, 90%, or 100% reduction in moderate/severe VMS; odds ratios for response were analyzed via logistic regression. Kaplan-Meier was used to estimate time to response, which was summarized descriptively. Least squares (LS) mean percent reduction from baseline in daily VMS frequency was determined with a mixed effect model repeated measures approach.
Results: Of 356 women randomized, 352 received ≥1 dose of study drug (safety population; mean [SD] age: 54.6 [4.7] y; 73% white) and 287 (81%) completed the study (placebo: 84%; fezolinetant: 80%). The percentage of patients with 50% reduction in moderate/severe VMS at last on-treatment assessment was significantly higher with fezolinetant 15, 30, 60, and 90 mg BID (83.7%, 81.4%, 88.1%, and 94.7%, respectively) or 30, 60, and 120 mg QD (82.1%, 88.1%, and 84.1%, respectively) than with placebo (58.5%; all P<.05 for odds ratio for response vs placebo). Mean time to 50% reduction in moderate/severe VMS ranged from 2.2–8.4 days with fezolinetant depending on dose, and 15.1 days with placebo (Figure 1). LS mean percentage reduction from baseline to week 12 in daily frequency of moderate/severe VMS were 74.3%, 75.8%, 80.2%, and 86.9% for 15, 30, 60, and 90 mg BID and 75.1%, 77.7%, and 76.9% for 30, 60, and 120 mg QD, respectively (common SE: ~5; all P<.01). Most TEAEs were mild/moderate. No deaths or serious treatment-related TEAEs occurred.
Conclusion: A significantly larger percentage of women treated with most doses of fezolinetant achieved treatment response, compared with placebo, and percentage reductions in daily moderate/severe menopausal VMS were significantly larger with fezolinetant. Kaplan-Meier estimates demonstrate a rapid onset, as early as the first few days.
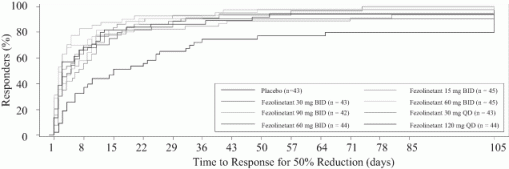
Figure 1 Kaplan-Meier Plot of Time to 50% Reduction in Moderate/Severe VMS.



