BES2021 Belgian Endocrine Society 2021 Abstracts (26 abstracts)
Thinking beyond guidelines: an atypical case of adrenal incidentaloma
Vliebergh Joke 1 , Van Cleynenbreugel Ben 2 , Vermeer Sascha 3 , Sciot Raf 4 & Bex Marie 1
1Department of Endocrinology, University Hospitals Leuven, Belgium; 2Department of Urology, University Hospitals Leuven, Belgium; 3Department of Center for Human Genetics, University Hospitals Leuven, Belgium; 4Department of Pathology, University Hospitals Leuven, Belgium;
Background: An adrenal incidentaloma is defined as an adrenal mass larger than 1 cm, detected on imaging performed for an indication other than evaluation of adrenal disease. Following the European Society of Endocrinology clinical practice guideline, assessment of malignancy by imaging and hormone excess should be done simultaneously(1). To investigate whether an adrenal mass is functionally active, a thorough clinical examination is required, extended with a 1mg overnight dexamethasone suppression test and measurement of plasma or urinary metanephrines to rule out autonomous cortisol secretion and pheochromocytoma respectively. Measurements of plasma-free or urinary fractionated metanephrines have an excellent sensitivity of 97% and specificity of 91% for the diagnosis of pheochromocytoma and are therefore recommended as initial screening tests(2). Chromogranin A (CgA) is also known as circulating marker of neuroendocrine tumors but is not routinely recommended to be measured.
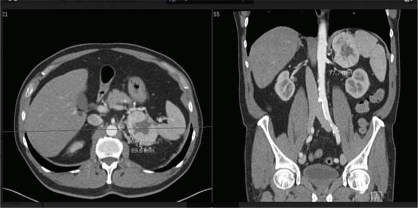
Clinical Case: A 65-year-old man was referred by the urologist after detection of an adrenal incidentaloma on CT abdomen performed in the work-up for recurrent prostatitis. His medical history further included discus herniation, hypercholesterolemia treated with a statin and arterial hypertension which was well controlled on triple therapy with an ACE-inhibitor, calcium-channel blocker and beta blocker. On examination he had a normal BMI and systolic arterial hypertension grade I. The mass was localized in the left adrenal gland, measured approximately 50 x 70 x 78 mm, had regular borders but a heterogeneous composition with a density of > 10 Hounsfield Units, central necrosis and focal calcification and was highly vascularized (figure). Based on these imaging features, adrenocortical carcinoma or pheochromocytoma was suspected. Initial hormonal work-up showed no arguments for autonomous cortisol secretion nor increased levels of sex or precursor steroids and an increased renin activity due to ACE inhibition. Serum CgA was also measured and was markedly elevated (1203 µg/L), favoring a diagnosis of pheochromocytoma, as the gastrin level was normal. Surprisingly, the urinary excretion of catecholamines and fractionated metanephrines was normal on repeat testing, resulting in the diagnosis not being biochemically confirmed. However, an additional Ga68-dotate PET-CT scan showed increased expression of somatostatin receptors in the left adrenal mass only, which excluded other neuroendocrine tumors that could explain the elevated CgA. Preoperative alpha adrenergic receptor blockade was started and an open left adrenalectomy performed. Pathological examination confirmed the diagnosis of a pheochromocytoma 50 x 74 x 73 mm with positive staining for CgA and synaptophysin, and some adjacent normal adrenal parenchyma. Genetic screening with a pheo panel including RET, VHL and SDH genes detected a class 5 heterozygous nonsense mutation c.508C> T (p.Gln170*) in the SDHA gene, causing a premature stop codon.
Conclusion: Strict following of the clinical practice guidelines would have missed the preoperative diagnosis of this rare non-secreting pheochromocytoma because of the lack of hypersecretion of urinary metanephrines, which is advised as the only screening test. When suspicion is high, measurement of CgA can be a useful additional marker for pheochromocytoma. Secondly, genetic analysis revealed a mutation in the SDHA gene, which is only since the last decade known as an additional susceptibility gene for mainly paraganglioma and pheochromocytoma. To our knowledge, this is the first report of a non-secreting pheochromocytoma associated with an SDHA gene mutation(3). The question rises whether this SDHA gene mutation could be the explanation for the rare biochemically silent character of this pheochromocytoma?
References: 1. Fassnacht M et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016 Aug;175(2): G1-G34.
2. Jacques W. M. Lenders et al. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 99, Issue 6, 1 June 2014, Pages 1915 1942.
3. Bausch B et al. European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017 Sep 1;3(9):1204-1212.
 }
}



