BES2023 BES 2023 Section (29 abstracts)
A non-controlled Cushing disease treated with osilodrostat: A case report
Depoorter L 1 , De Block C 1,2 & De Herdt C 1
1Department of Endocrinology-Diabetology-Metabolism, Antwerp University Hospital, Antwerp, Belgium.; 2University of Antwerp, Faculty of Medicine and Health Sciences, Antwerp, Belgium
Introduction: Cushing disease (CD) is a rare pathology and associated with serious complications and an increased mortality (standardized mortality ratio 2.8) (1). The mortality risk is twice as high (standardized mortality ratio 5.7) if CD is biochemically not well controlled. Transsphenoidal tumor resection is the first line treatment. However, the need for second line treatment is high, as 5-50% of patients in remission after surgery relapse and consist of medical therapy to lower cortisol levels (2). Currently available medication in Europe and their effectiveness are: cabergoline (35%), pasireotide (44%), ketoconazole (44%), metyrapone (66%) and osilodrostat (81%) of which cabergoline and ketoconazole are available in Belgium (3, 4). Ketoconazole has, however, important side effects of which the most common are: hepatotoxicity (11-19%) and gastro-intestinal intolerance (4-19%) (5). We present you a case of a young women with treatment resistant CD well controlled with osilodrostat.
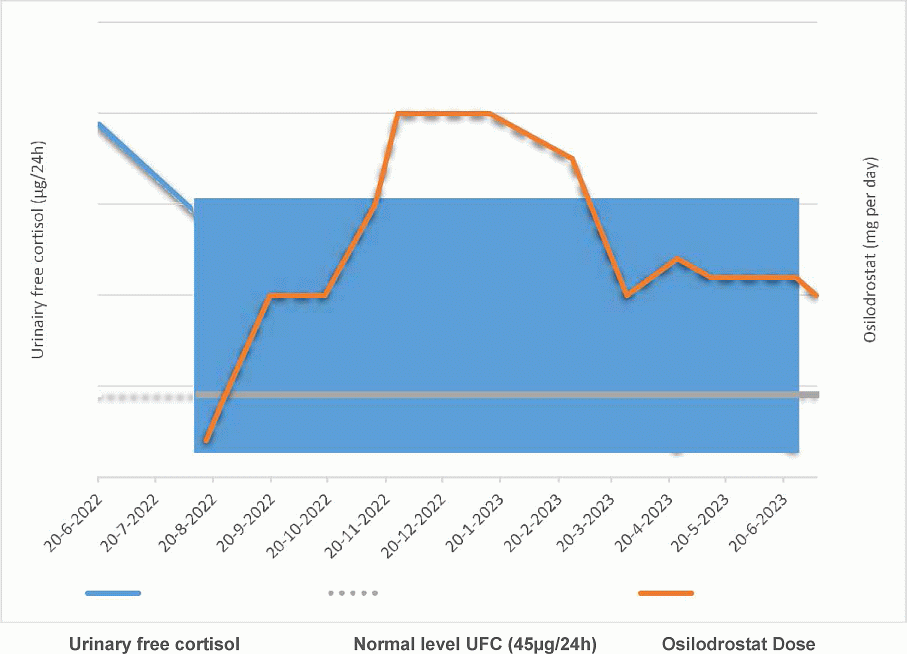
Figure 1. Evolution of the urinary free cortisol (UFC) level during dose titration of osilodrostat.
Case report: A 29-year-old woman was diagnosed with CD after developing spontaneous purpura, weight gain and oligomenorrhea. The laboratory findings showed a two-fold elevated prolactin (42,2 µg/l, <26.5µg/l) with a macro adenoma of 17mm on MRI pituitary. She was treated with cabergoline, resulting in a normalized prolactin level and shrinkage of the adenoma. Curiously, the 24h free cortisoluria (UFC) kept rising and the patient was diagnosed with CD for which she underwent twice a transsphenoidal adenectomy without achieving remission. In between the two surgeries the patient was treated with ketoconazole but experienced gastrointestinal side effects. Stereotactic radiosurgery (Gamma knife 60gy) was performed, but one year later UFC remained elevated (196 µg/24h, <45). Therefore treatment with pasireotide was started in a dosage of 20mg per month and was complicated by developing diabetes mellitus without improvement of UFC (178µg/24h, <45). Ultimately a bilateral adrenalectomy was suggested, which the patient refused. Osilodrostat was started in compassionate use. After 5 months of systematically increasing the dose to 20 mg daily, eucortisolism was achieved for the first time since diagnosis (in combination with pasireotide) (Fig 1). Patient felt better and diabetes mellitus improved (HbA1c 8 to 5.4%). MRI pituitary 3 months after commencement of osilodrostat showed no remnant growth. However, after 7 months of treatment she complained of joint pains and tiredness and morning cortisol level was decreased to a level of 78.2 µg/dl (ACTH 473 ng/l) for which the dose of osilodrostat was gradually decreased. Currently, the patient is treated with a dose of 11 mg per day.
Discussion: The need for second line treatment to achieve remission in CD is high, but currently available medication in Belgium (cabergoline and ketoconazole) is insufficient and complicated by side effects. Osilodrostat is a new oral medication that inhibits 11beta-hydroxylase, better known as the final step in adrenal steroidogenesis. In 2022, the results of a phase III study were published (LINC4) with inclusion of 73 patients with CD (4). Twelve weeks after treatment 77% treated with osilodrostat showed normalization of UFC compared to 8% in the placebo group. After 36 weeks of treatment, normalization of UFC was achieved in 81% of the total study population. Furthermore, osilodrostat has a beneficial effect on the cardiovascular and metabolic parameters leading to a decrease in weight, fasting plasma glucose, systolic and diastolic blood pressure, and cholesterol. Osilodrostat is generally well tolerated. The most common adverse effects were related to hypocortisolism; anorexia (37.5%), arthralgia (35.4%), and nausea (31.3%) and occurred mostly during the dose-titration phase. Osilodrostat is licensed in Europe for the use in CD but not reimbursed in Belgium. No head-to-head comparison between ketoconazole and osilodrostat exists but we conclude that in this particular patient case, osilodrostat had an added value in the treatment of CD.
References: 1. Limumpornpetch P, et al. The effect of endogenous Cushing syndrome on all-cause and cause-specific mortality. J Clin Endocrinol Metab. 2022; 107: 2377-388.2. Braun L, et al. Recurrence after pituitary surgery in adult Cushing’s disease: a systematic review on diagnosis and treatment. Endocrine. 2020; 70: 218-231.3. Galendi J, et al. Effectiviness of medical treatment of Cushing’s disease: a systemtic review and meta-analysis. Front Endocrinol. 2021; 12: 732240.4. Gadelha M, Bex M, Feelders RA, Heaney AP, Auchus RJ, Gilis-Januszewska A, Witek P, Belaya Z, Yu Y, Liao Z, Ku CHC, Carvalho D, Roughton M, Wojna J, Pedroncelli AM, Snyder PJ. Randomized Trial of Osilodrostat for the Treatment of Cushing Disease. J Clin Endocrinol Metab. 2022; 107: 2882-2895.5. Pivonello, et al. Cushing’s disease: adrenal steroidogenesis inhibitors. Pituitary. 2022; 25: 726-732
 }
}



