BES2024 BES 2024 CLINICAL CASE REPORTS (10 abstracts)
Arginine vasopressin deficiency associated with accelerated phase myeloproliferative neoplasm with monosomy 7
Mohamed Amine El Kesri 1 , Raluca Maria Furnica 1 , Martin Buysschaert 1 , Violaine Havelange 2 & Stefan Matei Constantinescu 1
1Department of Endocrinology and Hematology. 2Cliniques Universitaires Saint-Luc, 10 Avenue Hippocrate, 1200, Brussels, Belgium
Introduction : Arginine vasopressin (AVP) deficiency is defined by the inability to concentrate urine, secondary to a deficiency in antidiuretic hormone (ADH) production by hypothalamic neurons. The onset of this syndrome should prompt an extensive work-up, with a particular focus in elderly patients on a tumoral etiology affecting the posterior pituitary1.
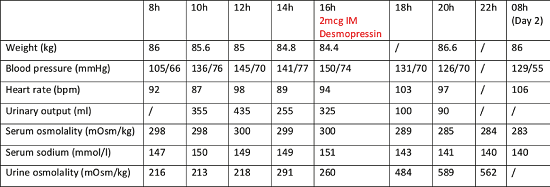
Case report: An 81-year-old female patient was referred to our endocrinology clinic for the evaluation of polyuria/polydipsia (approximately 3L/day of urine along with increased thirst and dry mouth) that had abruptly developed and persisted for one month. She was undergoing regular hematological follow-up for a myeloproliferative neoplasm and oncological follow-up for a breast carcinoma, treated in 2015 with neo-adjuvant chemotherapy and trastuzumab, followed by surgery, radiotherapy and adjuvant tamoxifen. Initial analyses performed by her diabetologist suggested AVP deficiency or resistance. The patient presented with a fasting glycaemia of 103 mg /dl (normal < 100 mg /dl) and HbA1c of 6.11% (normal 4-6%) high serum sodium levels of 144 and 147 mmol/l (normal 135-145 mmol/l) with normal serum osmolality of 287mOsm/kg (normal 275-295mOsm/kg) and low urinary osmolality of 191mOsm/kg (normal 300900mOsm/kg). The water deprivation test clearly indicated an AVP deficiency (Table 1). During the period of fluid restriction, the patient demonstrated a tendency towards hypernatremia and persistently low urinary osmolality, not reaching >600mOsm/kg as expected1. It was only after the administration of desmopressin that urine osmolality rose >50% and serum sodium normalized. Consequently, we initiated treatment with nasal desmopressin, which resulted in a good clinical response. Pituitary MRI revealed a slightly rightward-deviated pituitary stalk of normal thickness, a normal anterior pituitary and the absence of the spontaneous T1 hyperintensity of the posterior pituitary. 18F-FDG PET/CT did not show any suspicious hypermetabolic focus, and the pituitary gland did not exhibit increased uptake. A complete serological workup for autoimmune disease and germinoma was reassuring (including assessments of angiotensin converting enzyme, alpha-foetoprotein, human chorionic gonadotropin, complement, IgG4, and ANCA). Anterior pituitary function remained intact. Concurrently with the onset of her AVP deficiency, the patient developed bicytopenia with anemia at 8g/dL (normal 12-15g/dl) and thrombocytopenia at 90,000/mm3 (normal 150-450 000/mm3) along with 1% circulating blasts. A bone marrow biopsy revealed malignant transformation of her hematologic disorder into an accelerated phase myeloproliferative neoplasm with excess blasts at 12%, likely therapyrelated (epirubicin-cyclophosphamide chemotherapy in 2015). The blasts exhibited monosomy 7. Despite the poor prognosis, treatment with Azacitidine and Venetoclax was proposed, but the patient opted for supportive care only. Six months after diagnosis, she maintains normal sodium levels under desmopressin, a normal anterior pituitary function and receives bi-monthly hematology follow-up for iterative transfusions. Follow-up MRI after 6 months was proposed but cancelled at the patient’s request. A pituitary biopsy, which could have provided a definitive diagnosis, was not performed due to her thrombocytopenia, the risk of postoperative pituitary hormone deficiency and the lack of potential therapeutic consequences.
Discussion: We report a case of AVP deficiency concomitant with accelerated phase myeloproliferative neoplasm with blast excess and monosomy 7. This clinical presentation, extremely rare, is known to occur in the context of myelodysplasia/acute myeloid leukemia with blasts harboring monosomy 72. The mechanism of AVP deficiency and its relationship to this specific chromosomal aberration remains to be elucidated, with tumor infiltration of the posterior pituitary by the blasts and local hemorrhage being described in small histological series3. AVP deficiency in this context is associated with a worse hematological outcome but may recover after treatment of the underlying hematological disease by chemotherapy or bone marrow transplantation3. This case highlights the need of careful evaluation of elderly patients presenting with polyuria and polydipsia and of performing an extensive etiological work-up to exclude infiltrative and malignant disease.Table 1:Evolution of clinical and biological parameters during the water deprivation test. The period of fluid restriction started at 00h and continued for 16h. IM: intramuscular
Table 1: Evolution of clinical and biological parameters during the water deprivation test. The period of fluid restriction started at 00h and continued for 16h. IM: intramuscular
References: 1. Tomkins M, Lawless S, Martin-Grace J, Sherlock M & Thompson CJ. Diagnosis and Management of Central Diabetes Insipidus in Adults. J Clin Endocrinol Metab 2022 107 2701-2715. doi:10.1210/clinem/dgac381.
2. de la Chapelle A & Lahtinen R. Monosomy 7 predisposes to diabetes insipidus in leukaemia andmyelodysplastic syndrome. Eur J Haematol 1987 39 404-411. doi:10.1111/j.1600-0609.1987.tb01447.x.
3. Muller CI, Engelhardt M, Laubenberger J, Kunzmann R, Engelhardt R & Lubbert M. Myelodysplastic syndrome in transformation to acute myeloid leukemia presenting with diabetes insipidus: due to pituitary infiltration association with abnormalities of chromosomes 3 and 7. Eur J Haematol 2002 69 115-119. doi:10.1034/j.1600-0609.2002.02763.x.
 }
}



