BES2021 Belgian Endocrine Society 2021 Abstracts (26 abstracts)
Therapy-resistant hypocalcemia in auto-immune polyendocrine syndrome type 1
Verroken Charlotte 1 , De Smet Stephanie 1 , Kerrebrouck Marianne 1 , Creytens David 2 , T’Sjoen Guy 1 & Lapauw Bruno 1
1Department of Endocrinology, Ghent University Hospital, Ghent, Belgium; 2Department of Pathology, Ghent University Hospital, Ghent, Belgium
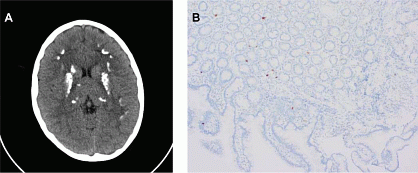
A 22-year old female is followed at our department because of genetically confirmed autoimmune polyendocrine syndrome type 1 (APS-1). This was diagnosed at the age of 9 and in this patient characterized by autoimmune hypoparathyroidism, autoimmune hepatitis, autoimmune gastritis and premature ovarian insufficiency. Throughout childhood and puberty, the hypoparathyroidism was treated with moderate doses of calcium citrate (~3g/d) and calcitriol (~1.5µg/d), nevertheless resulting in rather poor disease control with fluctuating but mostly rather low calcium values and the development of basal ganglia calcifications (Figure 1A). The autoimmune hepatitis, vitamin B12 deficiency and ovarian insufficiency were treated with azathioprine and budesonide, vitamin B12 injections and a combined oral contraceptive pill, respectively. Because of persistently low calcemia, calcium citrate and calcitriol doses were gradually increased and calcitriol was replaced by alfacalcidol between 2017 and 2020. Nonetheless, despite intake of 7g of calcium citrate and 6µg of alfacalcidol daily, calcium levels continued to decrease and in April, 2020, the patient was hospitalized because of severe hypocalcemia (1.29 mmol/l; albumin-adjusted) with symptoms including paresthesia, muscle cramps and fatigue. Given the underlying autoimmune syndrome, a trial with systemic glucocorticoids (methylprednisolone up to 64mg/d) was initiated. After 1 week, calcium levels gradually increased towards low-normal values and the calcium citrate and alfacalcidol doses could slowly be tapered towards 4g/d and 2µg/d, respectively. Unfortunately, attempts to taper the dose of methylprednisolone again resulted in decreasing calcium levels, and calcium citrate and alfacalcidol doses had to be reincreased. An underlying autoimmune form of malabsorption was suspected given the relatively good response to systemic corticosteroid therapy. Moreover, in addition to severe hypocalcemia, the patient had mild but chronic deficiencies in iron, magnesium and zinc stores for which she intermittently took supplements. Upon work-up, there were no arguments for coeliac disease based on repeated negative biochemical screening as well as negative intestinal biopsy in 2015, nor for the presence of autoimmune pancreatic insufficiency based on normal fecal elastase and absence of pancreatic autoantibodies. Furthermore, esophagogastroduodenoscopy and colonoscopy showed no arguments for colonization with Giardia lamblia or Candida albicans. However, reassessment of duodenal biopsy tissue from 2015 as well as new gastric, duodenal, ileal and colonic biopsies showed a reduced number of neuro-endocrine and especially serotonin-positive cells in the stomach and duodenum, suggesting a diagnosis of APS-1-associated autoimmune enteropathy (Figure 1B). In addition to systemic glucocorticoids, immunosuppressive therapy with rapamycin was started in November, 2020. However, therapeutic monitoring showed highly variable blood levels ranging from undetectably low to therapeutic despite minimal dose adjustments, suggesting compromised absorption. Moreover, calcium levels further decreased and the autoimmune hepatitis that had long been controlled started to flare up again, probably due to interruption of azathioprine when rapamycin treatment was started. In May, 2021, the patient was hospitalized again for a short course of high-dose intravenous corticosteroids (methylprednisolone 125mg/d for 2 weeks while maintaining rapamycin at 4mg/d, calcium citrate at 6g/d and alfacalcidol at 6µg/d). Under this regimen, calcium levels gradually increased towards the low-normal range (~1.9 mmol/l, albumin-adjusted), rapamycin levels increased towards the therapeutic range and liver tests normalized. At discharge, intravenous corticosteroids were replaced by oral methylprednisolone starting at a dose of 128mg daily. Since June, 2021, methylprednisolone doses are being tapered very slowly with weekly follow-up. Currently, the patient has stable calcium values and therapeutic rapamycin levels under methylprednisolone 64mg/d, rapamycin 3mg/d, calcium citrate 6mg/d and alfacalcidol 4µg/d. In the following weeks, we hope to further taper and stop methylprednisolone treatment and, if possible, decrease the high maintenance doses of calcium citrate and alfacalcidol. In addition, we plan to perform new intestinal biopsies to reassess the number of neuroendocrine and serotonin-positive cells to confirm (or refute) successful immunosuppressive therapy.
 }
}



